Fertilizer from the earth
UNFERTILIZED FERTILIZER

Fertilizer without fertilizers: new technology! It seems paradoxical, but it is! And practically for free! Is it possible to obtain fertilizers directly in the fields, during their processing? Yes, and here is why it can be done. As a result of electrohydraulic crushing of rocks and other materials, many chemical elements and their compounds included in their composition pass into water in the form of soluble compounds in quantities reaching 90-95% of their content in the original material. The number of elements passing into solution is estimated in tens. It is interesting to note that the poorer the given rock, the more intensively and with less energy expenditure the elements are released from it into the solution. Our soils from this point of view are poor "ores", but our needs are also small. After all, in order to provide a plant with microelements for a year, they are needed in very small quantities, and very little of the main elements of potassium, phosphorus and others are also required. From two or three handfuls of soil taken from any field, it is possible, with very little energy expenditure and using very simple means, to obtain fertilizers sufficient to feed plants on an area of 1 m² for a whole year. In addition, it is possible to obtain not all at once, but several times during the year, periodically fertilizing agricultural crops. "GrinBio 1000" is a unique solution that allows you to make complex fertilizers from ordinary soil! Soil from the field + water and after 30 minutes the finished fertilizers can be applied back to the field, without using any chemicals! We have a grandiose prospect: fertilizing fields ... with the fields themselves!
Peat Nitrogen analysis before and after
Earth Nitrogen analysis before and after

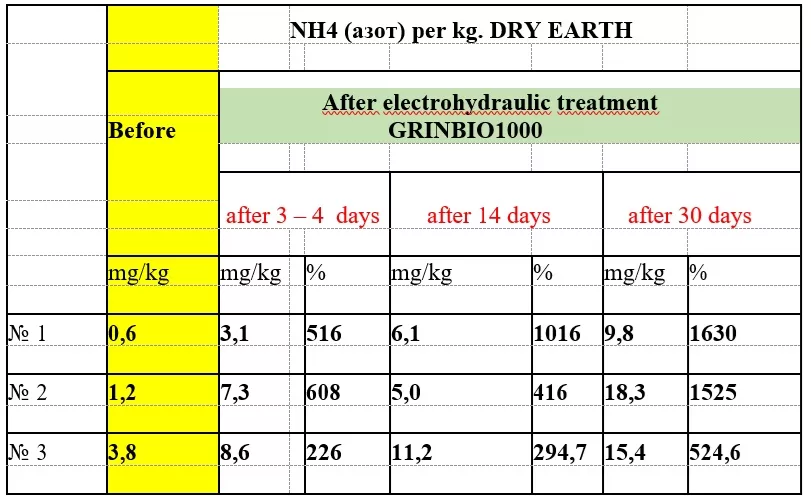
It should be noted that the analyses show nitrogen growth regardless of the treated soil or peat sample. All samples that we analyzed gave an increase in nitrogen. Some soil samples even showed an increase of more than 3000%. We determined that it is best to use different types of soil from different areas and mix them to get a homogeneous and quality fertilizer, as some areas are deficient in potassium and others are deficient in sodium or nitrogen. After mixing and appropriate processing in our GRINBIO-1000, the indicators of all micro and macro elements have increased several times, which makes the fertilizer from EARTH completely complete! After applying this fertilizer to the fields, the yield increase was from 30 to 50%.
Why is this happening? It’s all about  that EG-treatment (Electrohydraulic treatment) stimulates and sharply accelerates the course of chemical reactions. It was also established that in the soil mixed with water under the action of electrohydraulic shocks the content of O2 and O3 ions increases significantly, and a large number of OH anions intensively transform into hydrogen peroxide (H2O2), which then, decomposing into H2O and O, causes vigorous oxidation of the previously “passive” salts of the fertile layer by the formed atomic oxygen.
that EG-treatment (Electrohydraulic treatment) stimulates and sharply accelerates the course of chemical reactions. It was also established that in the soil mixed with water under the action of electrohydraulic shocks the content of O2 and O3 ions increases significantly, and a large number of OH anions intensively transform into hydrogen peroxide (H2O2), which then, decomposing into H2O and O, causes vigorous oxidation of the previously “passive” salts of the fertile layer by the formed atomic oxygen.
Under natural conditions, the overwhelming majority of complex soil salts can be considered insoluble in water, which, by the way, essentially does not so much dissolve as decompose or destroy them, carrying out this process extremely slowly, over tens of years. First, water “takes away” some part of the salt, converting it into simpler compounds. Then the remaining part of the still complex (no less than before) salt is again “simplified” under the further action of water. This continues until the initial soil salts are transformed into the final, simplest of the possible mineral compounds under specific conditions (SiO2, Al2O3, Fe2O3, etc.). But with electrohydraulic soil treatment, all the processes under consideration are sharply (up to hundredths of a second) accelerated, and they can also be made controllable, that is, their course can be selectively influenced.
An important factor is that the soil is a polydisperse system: its solid phases consist of particles of various sizes, from the largest ones – sand grains to colloidal particles with a diameter of several millimicrons. The role of highly dispersed colloidal fractions in creating the necessary soil conditions for plant development is very responsible and diverse. After all, they are the main suppliers of nutrients, since the availability of their absorption by plants and the ability of the soil to retain these substances is directly dependent on the size of the particles that make it up: the larger the specific surface area of the particles, the higher their nutritional properties. Thus, the leadership of silt soils in fertility is explained, in particular, by the fact that the total surface area of their particles reaches a huge value of 23,000 cm² per kilogram of soil.
As a result of electrohydraulic processing, almost the entire soil sample is crushed to particles close to colloidal or to colloidal particles, and their resulting total surface area can become significantly larger than even that of natural silt fractions. The resulting highly dispersed, very small particles actively interact with the compounds that have passed into solution, therefore, processes such as dissolution and especially sorption increase qualitatively and prove to be extremely effective. And large particles serve as a reserve fund, a soil reserve, due to which the electrohydraulic effect increases its overall dispersion. Obviously, several electrohydraulic handfuls of soil will be quite sufficient to satisfy the needs of plants for the necessary nutrients on one square meter of the field during the year.
But we haven’t said a word about nitrogen, which is the basis of a full-fledged “menu” of plants. This element is very common in nature, but plants often remain hungry, being, as they say, at a rich table. The EG effect can help here too. Experiments have shown that if ordinary irrigation water taken from any reservoir is processed electrohydraulic, “crushed”, the amount of dissolved nitrogen compounds in it quickly increases. In addition, air consisting of 78% nitrogen, gaseous nitrogen and even exhaust gases can be blown through it under low pressure, which, having been utilized in this way, will pollute the atmosphere less. And the result is amazing: ordinary irrigation water becomes a nitrogen-rich fertilizer!
Now we can say that the electrohydraulic effect can extract nutrients for plants directly from the soil, irrigation water and even air in the field, i.e. fertilize without fertilizers. And there is no need to worry about the reserves of “raw materials” – they are practically inexhaustible. However, if this is true for the most common in nature very poor lands, then, probably, EG-treatment will be especially beneficial for soils rich in nutrients, but, alas, very reluctantly, as we know, give them to plants. For example, peat, the deposits of which in our country are extremely large. It is not for nothing that they are called storehouses of the sun. Peat, indeed, as if accumulates solar energy in itself, becoming an excellent raw material for many sectors of the national economy. However, peat acquires fertilizing qualities only at a certain degree of decomposition, and this process in natural conditions is very slow. To speed it up, various methods are used – thermal, chemical, biological – that allow the organic matter to be converted into a state that is assimilable by plants.
The very first laboratory experiments showed the extreme efficiency of electrohydraulic processing of peat. It turned out that this leads to rapid decomposition of the organic matter of peat, bound forms of nitrogen and other nutrients, which become soluble, mobile, i.e. assimilable by plants. For example, the content of ammonia nitrogen increases depending on the type of peat by 1.5-5 times, and water-soluble organic matter by 1.5-6 times.
But the most amazing surprise was yet to come. Experiments revealed that the decomposition of the organic matter of peat and the increase in mobile forms of nitrogen, water-soluble carbon and other nutrients in it continues after electrohydraulic processing, during storage. Subsequently, based on the results of hundreds of experiments, it was established that free storage of electrohydraulic-treated peat at positive temperatures leads to a sharp (10-30 times!) increase in the content of nutrients assimilated by plants on the 10-15th day. For example, the dynamics of changes in the content (mg per 1 kg of dry mass) in peat is as follows: in its natural state 23.4 mg / kg, 3-4 days after EG-treatment – 73.6, and after 14 days already 760 mg / kg. And what is very important, in the future, peat practically does not lose its acquired fertilizing qualities. RESEARCHES WERE CONDUCTED IN OUR LABORATORY FOR 5 YEARS.
The results of the research on peat showed:
- Increase in the mass content of ammonia nitrogen by 1.4–4.5 times.
- Increase in water-soluble organic matter by 1.5–5 times.
- Hydrolytic deamination of free acids.
- Increase in the content of soluble nitrogen compounds by 5–10 times on the 10–15th day of storage.
Electrohydraulic processing of peat is:
- An effective and environmentally friendly solution for agriculture.
- A step towards the development of nanotechnology in the agro-industrial complex.
- Innovations for fertile lands and rich harvests.
- Increase in yield by 30-100%
- Reduce plant diseases by 80% without using chemical components
- Tillage of soil, plants without chemical fertilizers. Growing crops on a 100% biological natural basis.
INVENTION
The method of obtaining fertilizers from the ground using the Yutkin method was developed by the Soviet scientist Lev Alexandrovich Yutkin in the 30s of the last century. At that time, the method was not widespread, due to a complete lack of understanding not only of the processes, but also indifference in the systems of cultivation of crops by the BIO method. Moreover, in the 30s, no one conducted research on chemical fertilizers, as the chemical industry showed incredible development and of course the power of the USSR, despite 10 years of experiments and incredible growth rates of profit yields from 30 to 100%, did not allow the scientist to continue in this direction of additional research.
Moreover, because of his attempts to find investors abroad, he was recognized as a traitor and sent to prison.

How the GRINBIO-1000 method works
The principle of the method is that the electric discharge causes the breakdown of organic substances into simpler compounds that are more easily assimilated by plants.
 During the process of exposure to electric discharge, free radicals are formed, which initiate reactions of decomposition of organic substances. As a result of these reactions, amino acids, sugars, vitamins and mineral salts are formed.
During the process of exposure to electric discharge, free radicals are formed, which initiate reactions of decomposition of organic substances. As a result of these reactions, amino acids, sugars, vitamins and mineral salts are formed.
Why does this happen? The whole point is that EG treatment stimulates and dramatically accelerates chemical reactions. It was also found that in soil mixed with water, under the influence of electrohydraulic shocks, the content of O2 and O3 ions increases significantly, and a large number of OH anions intensively transform into hydrogen peroxide (H2O2), which then, decomposing into H2O and O, causes vigorous oxidation of previously “passive” salts of the fertile layer by the resulting atomic oxygen.
Under natural conditions, the vast majority of complex soil salts can be considered insoluble in water, which, by the way, essentially does not so much dissolve as decompose or destroy them, carrying out this process extremely slowly, over tens of years. First, water “takes away” some part of the salt, converting it into simpler compounds. Then the remaining part of the still complex (no less than before) salt is “simplified” again under the further action of water. This continues until the original soil salts are transformed into the final, simplest of the possible mineral compounds under specific conditions (SiO2, Al2O3, Fe2O3, etc.). But with electrohydraulic soil treatment, all the processes under consideration are sharply (up to hundredths of a second) accelerated and, in addition, they can be made controllable, that is, their course can be selectively influenced.
It is also important that the soil is a polydisperse system: its solid phases consist of particles of various sizes, from the largest – grains of sand to colloidal particles with a diameter of several millimicrons. The role of highly dispersed colloidal fractions in creating the necessary soil conditions for plant development is very responsible and diverse. After all, they are the main suppliers of nutrients, since the availability of their absorption by plants and the ability of the soil to retain these substances is directly dependent on the size of the particles that make it up: the larger the specific surface area of the particles, the higher their nutritional properties. Thus, the leadership of silty soils in fertility is explained, in particular, by the fact that the total surface area of their particles reaches a huge value of 23,000 cm² per kilogram of soil.
 As a result of electrohydraulic processing, almost the entire soil sample is crushed to near-colloidal or colloidal particles, and their resulting total surface area can become significantly larger than even that of natural silt fractions. The resulting highly dispersed, very small particles actively interact with the compounds that have passed into solution, so processes such as dissolution and especially sorption increase qualitatively and prove to be extremely effective. And large particles serve as a reserve fund, a soil reserve, due to which the electrohydraulic effect increases its overall dispersion. Obviously, several electrohydraulic processed handfuls of soil will be quite sufficient to satisfy the needs of plants for the necessary nutritional elements on one square meter of the field during the year.
As a result of electrohydraulic processing, almost the entire soil sample is crushed to near-colloidal or colloidal particles, and their resulting total surface area can become significantly larger than even that of natural silt fractions. The resulting highly dispersed, very small particles actively interact with the compounds that have passed into solution, so processes such as dissolution and especially sorption increase qualitatively and prove to be extremely effective. And large particles serve as a reserve fund, a soil reserve, due to which the electrohydraulic effect increases its overall dispersion. Obviously, several electrohydraulic processed handfuls of soil will be quite sufficient to satisfy the needs of plants for the necessary nutritional elements on one square meter of the field during the year.
Advantages of the method
GRINBIOS-1000 fertilizers have the following advantages:
- Contain a full range of nutrients necessary for plants.
- Fertilizers, natural, biological, complex, mineral from simple soil and water!
- Completely biologically clean.
- 100% Safe for the environment.
- Easy to manufacture.
- Almost free!
- The availability of raw materials right under your feet, any field is an unlimited raw material base!
- Mobility and ease of management!
- You can use: plain soil, peat, manure, sewage sludge, bird/chicken manure, brown coal, sapropel, lignin, river or sea silt, seaweed, crushed rock, granite, stone, hard minerals, sludge from biogas plants.
Disadvantages of the method
Fertilizers by the Yutkin method have the following disadvantages:
- Special equipment GRINBIO-1000 is required!
- The manufacturing process requires a careful approach and operator control.
Application of GRINBIO-1000 fertilizers
GRINBIO-1000 fertilizers can be used to feed any plants, both in open ground and in greenhouses. They are especially useful for plants suffering from a lack of nutrients.
Fertilizer from the earth can be applied to the soil at any time of the year, and if diluted with water, it can be used as a permanent watering.
Water that has received all the minerals and nutrients from the earth is an excellent means for permanent natural fertilization of plants and crops.

Storing fertilizers
Fertilizers made by our GRINBIO-1000 method,
can be stored in a dry, dark place for one year, but before use It is necessary to perform a nitrogen minimization, since during storage the amount of nitrogen increases to maximum values.
Environmentally friendly
Our GRINBIO-1000 Fertilizers are environmentally friendly. They do not contain harmful impurities and do not pollute the soil.
Conclusion
GRINBIO-1000 fertilizers are an effective and safe way to feed plants. They contain a full range of nutrients necessary for plants and do not contain harmful impurities. Fertilizers can be used to feed any plants, both in open ground and in greenhouses.
Subscribe to our newsletter
Subscribe to our newsletter to receive special offers and exclusive news about GrinBio products
About our fertilizers
My Account
Copyright © 2024 GrinBio. All rights reserved.
